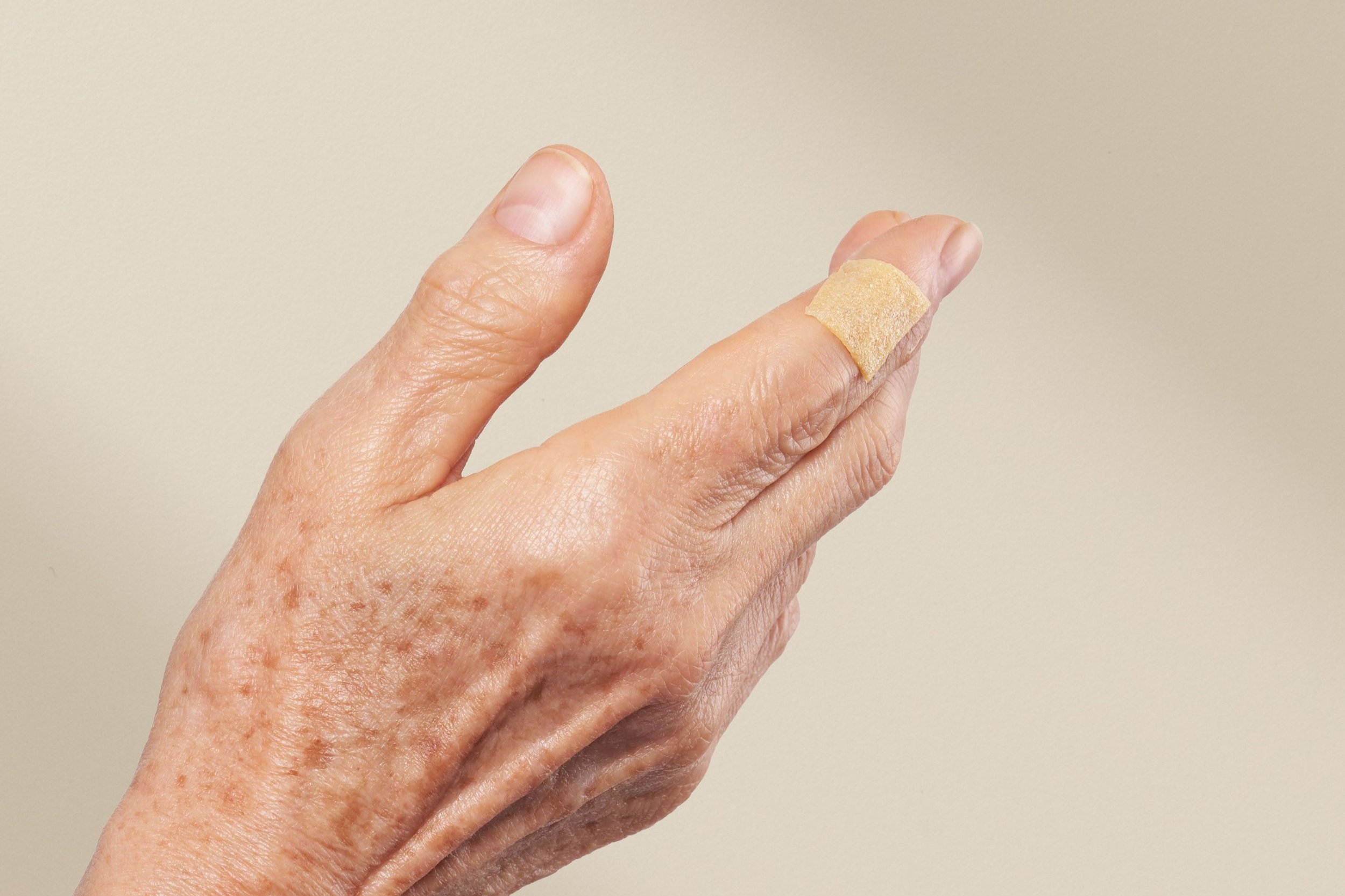
Recovery Starts with VERIS™
Wounds like skin cancer removal and diabetic ulcers can take months to close and can be painful. Made with collagen and certified Mānuka honey, VERIS™ helps patients recover from wounds in an average of 4-8 weeks and with lower pain. (1-4*)
The VERIS™ Difference
-
VERIS™ is a non-sticky solid sheet that dissolves daily, as the wound progresses.
-
VERIS™ is easy to use and ships directly to your home with all the supplies you need for recovery.
-
VERIS™ is powered by familiar ingredients like collagen and Mānuka honey, known to help wounds close.
-
VERIS™ is covered by Medicare and, depending on your policy, may be covered by commercial plans.
A One-of-a-Kind Solution Trusted by Doctors
Supported by published data, VERIS™ addresses the toughest barriers to wound healing and is prescribed by Mohs surgeons, Dermatologists, and Podiatrists across the country.
MATERIALS YOU CAN COUNT ON
-
A natural extracellular matrix protein, an essential part of organic tissue known to help control inflammation. (8)
-
Known for antibacterial properties and aiding in wound healing. (9)
-
A naturally occurring mineral known to add structural reinforcement to biomaterials and promote wound healing via angiogenesis and re-epithelialization. (10)
The easy-to-use packaging of VERIS™ has made self-application convenient, even while traveling, allowing me to maintain my wound care routine without hassle.
— VERIS™ PATIENT
Take Control with VERIS™
-

Talk to Your Healthcare Provider About VERIS™
If VERIS™ is right for you, your healthcare provider will prescribe and order VERIS™ for you to care for your wound at home.
-

Get Everything You Need Directly to Your Home
Just like a mail-order prescription, VERIS™ arrives at your home with with all the supplies needed and step-by-step directions.
-

Focus on Your Recovery Without the Hassle
Use VERIS™ as directed. For easy maintenance, VERIS™ dissolves daily as the wound progresses with no painful removal process.
VERIS™ is Easy to Use
-

Clean the wound
-

Hydrate VERIS™
Hydrate VERIS™ with saline.
-

Apply VERIS™
Gently press to ensure it contacts the wound.
-
Cover
Cover with bandage.
Repeat these steps daily or as directed by your physician.
FAQs
VERIS™ Overview
-
VERIS™ is FDA-cleared for the management of wounds, including chronic ulcers and surgical sites. VERIS™ is most effective on open, cleaned wounds, e.g. a debrided DFU or Mohs (skin cancer removal) wound left for secondary intention healing.
-
VERIS™ must be prescribed by your doctor. To support the conversation with your doctor, follow these two steps:
Download or share link to VERIS™ informational materials found here.
Have your doctor contact us at info@sweetbio.com or 888-323-2015 and our team will respond within 24 hours.
-
Powered by a patented combination of 3 natural ingredients, VERIS™ is a one-of-a-kind solution backed by science. Ingredients:
Collagen Derivative: A natural extracellular matrix protein, an essential part of organic tissue. (8)
100% UMF™-Certified New Zealand Mānuka Honey: Known for antibacterial properties and aiding in wound healing. (9)
Hydroxyapatite: A naturally occurring mineral known to add structural reinforcement to biomaterials and promote wound healing via angiogenesis and re-epithelialization. (10)
-
VERIS™ was created by SweetBio, Inc. Learn more about SweetBio's mission and values here.
-
VERIS™ is made with SweetBio®’s technology, MX™, which addresses the wound's microenvironment by balancing key variables known to heavily impact both early stages (i.e., homeostasis and inflammatory phases) and late stages (i.e., proliferation and remodeling phases) of the wound healing process. (5-7*)
-
VERIS™ is covered by Medicare as a wound care supply. Depending on your policy, VERIS™ may be covered by commercial plans.
-
There is no FDA-cleared claim for VERIS™ or any other Mānuka honey product on the market as an antibacterial product. However, Mānuka honey is well-researched for its antibacterial properties, and SweetBio®’s MX™ technology has demonstrated a remarkable 99.99% reduction in gram-positive and gram-negative bacteria in vitro, which is published in peer-reviewed journals. (5*)
-
Yes, the Mānuka honey used in VERIS™ is 100% UMF certified and medical grade, which is filtered and sterilized.
Tips for the best VERIS™ experience
-
Since VERIS™ is made with natural ingredients, you might notice variations in shape, size, or color from one side to the other. That’s completely normal—and either side can be placed on the wound.
-
Several drops of saline are sufficient to moisten VERIS™. This helps it soften, conform to the wound, and support a moist healing environment. VERIS™ should be wet, not soaking or dripping, to avoid saturating the covering bandage. Note that VERIS™ may expand slightly as it hydrates with saline.
-
You might notice a light scent when opening the pouch. It’s from the protein base of collagen and will fade within seconds.
-
VERIS™ must contact as much of the wound as possible and does not need to overlap the edges of the wound. Gently press to ensure it contacts the wound.
-
On average, VERIS™ dissolves daily. Apply a new VERIS™ product per your clinician’s recommendation.
-
If VERIS™ hasn’t fully dissolved when changing the bandage, do not remove it— simply add a few drops of saline and cover with a new bandage.
-
VERIS™ is not indicated for:
Patients with sensitivity to collagen and its derivatives
Patients with sensitivity to porcine-derived products
Patients with sensitivity to honey
Third degree burns
Infections or contamination in the wound site
Control of bleeding
Children and infant patients under 10kg / 22lbs
Usage Information
Users should follow the most recent procedures and best practices for wound management to complete wound preparation, application of VERIS™ and application of secondary dressings.
Remove VERIS™ and discontinue use if signs of sensitivity appear.
Do not use VERIS™ after the expiration date stated on the product label.
Contact a health care professional if:
Signs of infection occur (e.g. increased pain)
There is a change in wound color and/or odor
The wound does not begin to show signs of healing, or
Any other unexpected symptom
We’d love to hear from you!
The safety and well-being of our patients and those that care for them is what matters most. You can count on us through every step.
CITATIONS
1. Rodriguez et al. Novel bioengineered collagen + Manuka honey + hydroxyapatite sheet for the treatment of lower extremity chronic wounds in an urban hospital wound care setting. 2023.
2. Rodriguez et al. Preliminary clinical evaluation of a novel bioengineered wound product: A focus on the management of lower extremity ulcers. 2020.
3. McMurray et al. Use of a Novel Biomaterial to Enhance Secondary Intention Healing. 2021.
4. Arnaud et al. Novel Biomaterial Containing Gelatin, Manuka Honey, and Hydroxyapatite Enhanced Secondary Intention Healing Versus Standard Secondary Intention Healing in Mohs Surgical Defects on the Head and Distal Lower Extremities-A Randomized Controlled Trial: Pilot Study. 2023.
5. Williams et al. A novel bioengineered wound product with in vitro capabilities to reduce bacteria. 2021.
6. Rodriguez et al. Microenvironment influence of a novel bioengineered wound product APIS®: An in vitro analysis of inflammatory marker and growth factor secretion. 2021.
7. Rodriguez et al. A Preliminary Direct Comparison of the Inflammatory Reduction and Growth Factor Production Capabilities of Three Commercially Available Wound Products: Collagen Sheet, Manuka Honey Sheet, and a Novel Bioengineered Collagen Derivative + Manuka Honey + Hydroxyapatite Sheet. 2022.
8. Dantas et al. 2011; Li et al. 2014; Pezeshki-Modaress et al. 2014; Pezeshki-Modaress et al. 2015
9. Sell et al. 2012; Afrin et al. 2018; Johnston et al. 2018; Minden-Birkenmaier et al. 2018
10. Okabayashi et al. 2009; Majeed et al. 2012; Kawai et al. 2011; Weilin et al. 2016; Tommila et al.2008; Vilardell et al. 2016; Rodriguez et al. 2013 & 2016
* Based on clinical and in vitro data; claims have not been cleared by the FDA. Faster wound closure reported in slower healing and chronic wounds compared to standard of care.


